

ISO 5: Defining Cleanroom Classification Guidelines
ISO 5: Defining Cleanroom Classification Guidelines
A space where other environmental factors may be measured (humidity, temperature, pressure)Design: An enclosed space constructed in a way to minimise the introduction, generation and…
What Is A Cleanroom?
One of the leading UK cleanroom training boards, ATI define a cleanroom through 2 areas.
Control: A space where the number of airborne particles are measured. A space where other environmental factors may be measured (humidity, temperature, pressure)
Design: An enclosed space constructed in a way to minimise the introduction, generation and retention of particles.
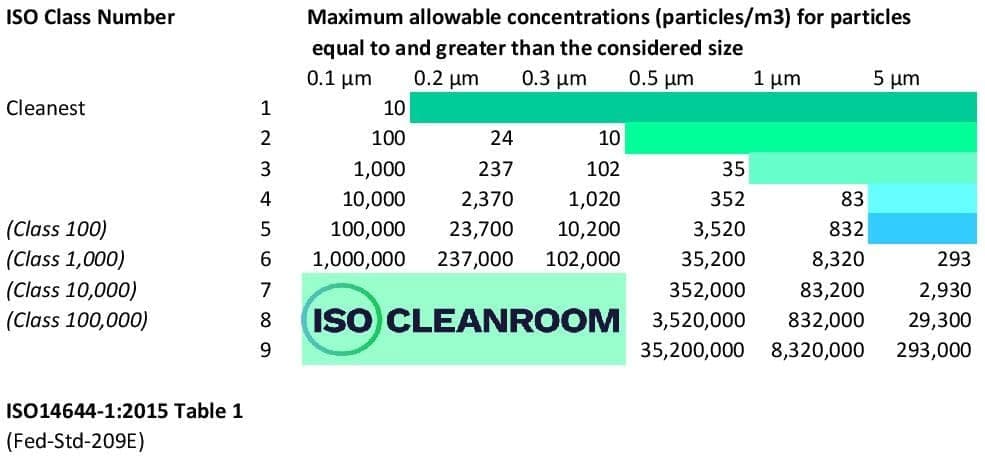
There are 9 ISO classifications, ISO 1, ISO 2, ISO 3, ISO 4, ISO 5, ISO 6, ISO 7, ISO 8, and ISO 9. ISO 1 is considered the cleanest while ISO 9 is considered the dirtiest. The basis of cleanroom standards is the micrometer or micron. Each ISO classification allows a certain maximum number of particles per m3 of air.
This table of the ISO14644 series best illustrates the maximum concentration limit per cleanroom class.
What is an ISO 5 Cleanroom?
You can see from the table above that for a space to be classified as an ISO 5 cleanroom, it must contain less than 832 particles > 1 micron, 3,520 particles > 0.5 micron, 10,200 particles > 0.3 of a micron and so on per cubic meter.
When deciding which classification you require, you should ask what size of particle can negatively affect your procedure or product. The answer will be unique to your process and is key to understand prior to designing your cleanroom.
Why Do You Need an ISO 5 Cleanroom?
Typically, semi conductor, advanced engineering, optics, laser and electronics require an ISO 5 cleanroom along with pharmaceutical and medical devise companies. The reasons that companies require these facilities vary from yield (money) to safety (regulation).
Semi conductor industries tend to be concerned with yield as contamination via particles as small as 0.1 of a micron can render the work unusable at best or potentially dangerous. Pharmaceutical and medical devise companies require sterile environments to ensure that their products are safe. They will be concerned with not only the size and number of particles in the room but also what those particles are. Are they viable or a biological particle. (Bacteria, spores, fungi etc) or no-viable particles (dead skin cells, dust from mechanical parts in the cleanroom, fibres from clothing etc)? Further tests are required in these cleanrooms to determine the make up of the particles.
How To Get an ISO 5 Cleanroom?
The design criteria to achieve these standards consider the quality of air, the quantity of air and the movement of air. ATI have summarised it in the following way;
• The air supplied to a cleanroom is of the correct quantity to dilute or remove contamination
• The air supplied is the correct quality
• The air in the cleanroom suite moves in the correct way i.e. from clean to less-clean.
• Air movement within the cleanroom ensures that there are no high concentrations of contamination.
An ISO 5 cleanroom could use non-unidirectional airflow or unidirectional airflow to achieve the above criteria. The amount of complete volume air changes per hour in an ISO 5 cleanroom is 240+ all of which will pass through either High efficiency HEPA filters or Ultra Low Penetration ULPA filters depending on the identified problem particle size.
H14 HEPA filters have a 99.995% efficiency at 0.3 micron.
U15 ULPA filters have a 99.9995% efficiency at 0.12 micron.
How to Maintain an ISO5 Cleanroom?
Maintaining an ISO 5 Cleanroom requires extensive care. In order to keep at this level a complete monitoring process must be implemented. The monitoring will change between industries and applications but there are a few fundamental points
- Monitoring of particulate.
- Constant positive pressure.
- A material selection protocol for all items permitted into the cleanroom
- All personnel wearing PPE
- Regular checks of the plant including filter changes.
Do’s and Don’ts Inside a Cleanroom
- Wipe all surfaces regularly to remove contamination
- Keep all doors closed to maintain positive pressure
- Clean all equipment that’s being brought inside the cleanroom
- Have all personnel wearing personal protective equipment (PPE) over their clothes before going inside the cleanroom
- Don’t bring unnecessary objects inside the cleanroom
- Don’t eat or drink in your cleanroom
- Don’t turn off the HEPA fan filter units
Are you looking for a cleanroom solution? Contact us now to know more!